But still nothing is comparable to down south! What's the problem, Vancouver? It may be with the roasting - green, astringent-tasting raw coffee beans should be transformed into the familiar brown, aromatic beans, although up to a temperature of 150 °C the beans simply lose water; true roasting begins only above 160 °C when chemical reactions - incalculable in number - take place, and the constitution of the beans changes with the principal product being in fact carbon dioxide (for every kilogram of beans, as much as 12 L of CO2 will be released) but since during the roasting process the very thick cell walls of the beans remain intact, released CO2 causes pressure within the cells to increase to as much as 25 bar - in other words, the chemical roasting reactions take place between 160 and 240 °C in tens of thousands of mini-autoclaves so it should come as no surprise that, under these harsh conditions, thousands of new compounds are produced in the course of thermal decomposition of the over 700 so-far identified components of green coffee beans, as well as of the many polymeric storage and skeletal components meaning that from a chemical standpoint coffee is actually the most complex beverage we consume where most reactive constituents are free amino acids and simple sugars like glucose, galactose, and arabinose, as well as the disaccharide saccharose though, with increasing temperature, trigonelline and the chlorogenic acids are largely decomposed as well, whereas lipids and caffeine are nearly unaffected by roasting (chlorogenic acids are esters comprised of quinic acid as the alcohol part and a p-substituted p-hydroxycinnamic acid as the acidic component - "chlorogenic" stems from a green color observed in the course of its alkaline oxidation, a reaction discovered in the 19th Century) and the various brown to black pigments arise through a confusing reaction cascade, still not clarified in detail, in which simple sugars like glucose and arabinose form caramel-like products that can in turn react further with chlorogenic acids to give red to brownish-black humic acids while, parallel to this, free amino acids react with the saccharides by way of Mailard reactions to yield yellow to brownish-black melanoidins meaning that, overall, pigment formation involves substances in every compound class, with the exception of caffeine and the fats, allowing the roasting process to play a decisive role with respect to both aroma and flavor; espresso could in principle be prepared from any coffee roast but the more darkly roasted beans are preferred, in which components have undergone more complete thermal decomposition and, as a consequence, the proportion of astringent-tasting chlorogenic acids is decreased, which explains the softer taste of espresso relative to less strongly roasted coffees while trigonelline is also heavily decomposed, producing a multitude of heterocyclic compounds, which in turn contribute to the powerful roasted aroma (it is worth noting the development in the process of the vitamin nicotinic acid (niacin) - drinking a cup of espresso actually supplies roughly 15% of the recommended daily dose of this vitamin!) 1
Problems could also arise through the preparation of the shot: passage of a solvent (hot water) through a solid phase (coffee powder) under pressure is very simple from an apparatus perspective, and is reminiscent in some ways of high-pressure liquid chromatography (HPLC) so for laminar flow of a solvent through a cylindrical column (radius r, length L) filled with porous particles (diameter d), Darcy’s law permits derivation of the following expression for approximating the relationship between pressure difference and volume velocity V/t:
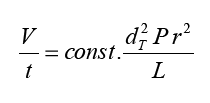
Such boundary conditions as amount of coffee, water temperature, diameter of the filter, applied pressure, and extraction time have been optimized empirically in thousands of Italian espresso bars over decades, while a glance through a microscope reveals that grist from the coffee mill is not homogeneous so that under the applied pressure as a water front moves it carries with it the smallest coffee particles, which then travel past the larger ones to congregate at the bottom of the layer of coffee powder, the resulting partial blockage leads to an increase in hydraulic resistance, and the flow velocity decreases and, if the flow direction is now reversed, small particles again move in the flow direction so at first the hydraulic pressure decreases, because the “blockage” disappears until small particles once again collect - at the other end - and hydraulic resistance increases once more but the chemical processes occurring in an espresso machine are even more complex: during the brief extraction period, equilibrium cannot be established between the phases, and only 75 % of the highly soluble caffeine is extracted therefore this incomplete extraction would at first appear to be a shortcoming, but in fact perfection lies in this defect: many components with undesirable sensory effects are left behind, as a result of which espresso is more readily digestible than ordinary brewed filter coffee and it is not just water-soluble compounds that are extracted; the hot water also causes the melting of lipids that have diffused to the surface after roasting, and the rapid streaming between coffee particles leads to formation of a fine lipid emulsion, with drop sizes between 0.5 and 1.0 µm and dissolved in these fat droplets are aromatic substances that would otherwise evaporate upon departure of the hot liquid but there is no need to worry - the fat content of espresso is very low, and even those obsessed with such things have absolutely no reason to suffer a guilty conscience over a mere 9 kcal. 2
But my feeling is that the baristas around here lack skill with their milk steaming, specifically texturing but also heating to a level that slightly cooks the lactose. Milk is a complex liquid, with fats, proteins and sugars, the main protein being casein; a long, string like molecule, which is folded up tightly, just like a ball of wool, very good for health because it’s a complete protein that has all the 20 amino acids the body needs for growth and these amino acids come in two types, some of them dissolve in water easily, while some don’t like water and normally the water-hating amino acids are inside the tightly wound up string but when boiling milk the casein unwinds, and all amino acids face the water so it acts just like soap, with parts that dissolve in water and parts that repel it while the sweetness we get from milk has to do with the lactose, although in reality lactose is a combination of two sugar molecules, galactose and glucose, held together is suspension as it is not very soluble, so the lactose stays in suspension in milk liquids and while cold it does not taste extremely sweet but as the lactose is heated the solubility breaks down and the sweetness of our beverages increases and such is the case with milk being steamed in an espresso machine where the steam breaks down the suspension-ability of the lactose releasing the sugars into our cups, meanwhile as you add steam to milk it is the amino acids that stick to the outside of the air molecules and build a cage around each and cause it to ‘float’, protect it and give it stability within our drinks although the fat content of the milk greatly affects how proteins react during this process, as does the temperature, because fat is influenced by temperature which influences the proteins, therefore although it is easier to get nice foam with skimmed milk than it is with whole milk, as the fat content continues to rise foam volume and stability go up once again so you’ll see high foam in a cream with about 10% fat while whipping cream is 35% fat: volume and stability. 3
But I'm just guessing really. Off for another cup!
Café Artigiano #1: no.
Café Artigiano #2: better.
Finch's Tea and Coffee: next time I'll order tea.
Two wheels on my wagon - half-way through our tour, Pete's stroller wheel burst! I went to Simon's Bike Shop and a man (Simon?) replaced the ruptured inner tube and refitted the wheel in seconds. Pete slept through the whole thing. "You're a magician!" I told the man. "No, just a professional," he humbly replied. A baby's snores begged to differ.



